
|
|

|
|
| April 25, 2024 |
|
Two more hospitals report 'superbugs' on endoscopes 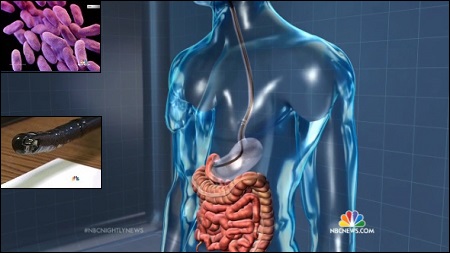
Two more hospitals, one in California and one in Connecticut, reported they'd found drug-resistant "superbugs" on a hard-to-clean type of medical equipment that's under federal scrutiny.
The devices, called duodenoscopes, have been modified in a way that allows germs to stick to them, the Food and Drug Administration says. They've been blamed for an outbreak of drug-resistant bacteria that's killed at least two patients and infected five more at UCLA's hospital. The university has warned about 180 people that they may have had the same contaminated scope used on them. Now Cedars-Sinai Medical Center, also in Los Angeles, says it's found the same type of carbapenem-resistant enterobacteria (CRE) in four patients who had procedures using duodenoscopes. The scopes, flexible tubes that carry a camera and other equipment into the body through the mouth—are specifically designed for procedures called endoscopic retrograde cholangiopancreatography (ERCP). They are used to treat patients with pancreatic cancer and other serious illnesses. "Despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA, the medical center's infection-control specialists announced today that their investigation has identified a total of four patients who had a CRE transmission (carbapenem-resistant enterobacteriaceae) linked to an ERCP procedure," the hospital said in a statement. "The same duodenoscope was used in all four patients, whose ERCPs occurred between August 2014 and January 2015." Separately, Hartford HealthCare said it had found a rise in cases of a different "superbug" but also linked it to the scopes. "A routine monitoring of cultures done at Hartford Hospital identified an increase in extended spectrum Beta-lactamase E. coli (ESBL), a drug resistant version of E. coli. After further review, we determined that this increase may have been associated with having the endoscopic retrograde cholangiopancreatography (ERCP) procedure," the hospital's chief medical officer Dr. Rocco Orlando said in a statement. At the facility, some 281 patients who underwent certain endoscopic procedures over the past year were potentially exposed to a drug-resistant form of E. coli, hospital officials said. "We do not believe any of our patients are in danger, but because safety is one of our core values, we are reaching out to every patient who has undergone this procedure and may have come in contact with this version of e-coli. We have been proactive, calling patients this week to alert them, and asking them to come in for screening," it added. "We want to assure you that while the process for cleaning our endoscopes was followed, due to the same design flaw we are seeing across the country, there is a section of the scope that is difficult to disinfect. We are no longer using the scopes and have pulled them from every hospital across our system." Cedars-Sinai says it has also removed the suspected device and warned 71 patients who were treated with it. The FDA says it's working with the three companies that make the devices to get them approved and to find better ways to clean them. One mechanism at the tip has microscopic crevices that a brush cannot get into. "Residual body fluids and organic debris may remain in these crevicesafter cleaning and disinfection," the FDA said in a notice sent out to medical professionals last month. The three companies told FDA they did not know that the changes they made to the devices meant they needed a new approval from the agency. FDA says about 500,000 procedures are done using the devices and says the small risk of infection is worth the benefit of using the devices. The patients otherwise would have to have open surgery, which is much riskier. Olympus Corp.'s U.S. unit was sued by Los Angeles hospital patient Aaron Young, who says he was infected. "Olympus continues to monitor and investigate this issue. We are working with the FDA, relevant medical societies and our customers regarding these concerns including the consideration of alternative cleaning and reprocessing methods," the company said in a statement. (Source: NBC News) Story Date: March 8, 2015
|